Introduction
As a medical oncologist, I recently attended a major congress where groundbreaking advancements in colorectal cancer (CRC) screening were presented. The insights shared at the event highlighted both current methodologies and emerging technologies that promise to revolutionize how we approach CRC detection and prevention. Here, I summarize these findings and their implications for clinical practice.
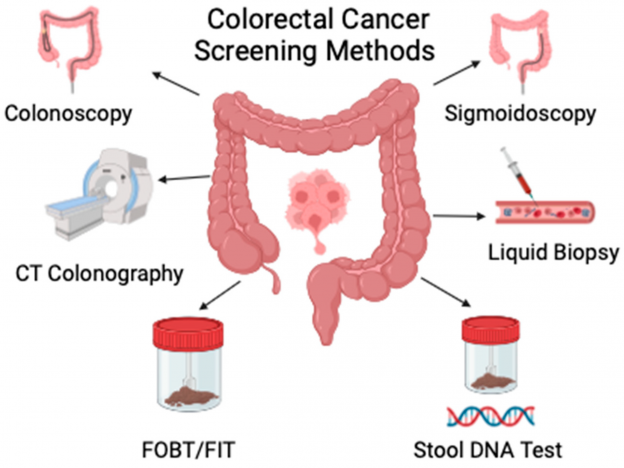
Current Screening Landscape
Colorectal cancer remains a significant global health challenge, making effective screening strategies more crucial than ever. The congress underscored several established screening methods:
- Colonoscopy: Widely regarded as the gold standard, colonoscopy allows for the visualization and removal of polyps throughout the colon. Its role in reducing CRC incidence and mortality is well-documented.
- Stool-Based Tests:
- Fecal Immunochemical Test (FIT): This test detects occult blood in stool samples and is praised for its non-invasive nature and cost-effectiveness.
- Fecal Occult Blood Test (FOBT): Utilizes a chemical process to identify blood in the stool, serving as an alternative to FIT.
- Stool DNA Testing: By analyzing genetic mutations in stool samples, this method offers a promising avenue for early detection.
- Flexible Sigmoidoscopy: Focuses on the lower part of the colon and is less invasive than a full colonoscopy.
- CT Colonography: Also known as virtual colonoscopy, this imaging technique provides a comprehensive view of the colon without the need for traditional endoscopy.
Emerging Technologies in CRC Screening
The congress also showcased innovative screening technologies that are poised to enhance our diagnostic capabilities:
- Blood-Based Biomarkers:
- cfDNA Tests: The FDA-approved Guardant Shield™ test detects circulating tumor DNA in the bloodstream, offering a convenient and non-invasive screening option.
- cfDNA + Protein Tests: Freenome’s approach combines cfDNA with protein biomarkers to improve detection rates, particularly for early-stage CRC.
- Advanced Stool Tests:
- MT-sRNA Test: Developed by Geneoscopy, Colosense™ utilizes stool-derived RNA markers to identify CRC with high sensitivity.
- MT-sDNA Test: Exact Sciences’ Cologuard Plus™ integrates multiple DNA markers to enhance detection accuracy, representing a significant advancement over traditional stool tests.
Comparative Analysis of Screening Methods
A comparative analysis presented at the congress highlighted the strengths and limitations of various screening methods:
| Method | Sensitivity for CRC | Specificity | Key Features |
|---|---|---|---|
| FIT | 67%-91% | 95% | Non-invasive, cost-effective |
| Cologuard Plus | 95% | 91%-94% | Multi-target stool DNA test |
| Colosense | 94% | 88% | RNA markers + FIT |
| Shield™ or Freenome | 80.5%-83% | 90% | Blood-based cfDNA test |
Challenges and Limitations
While the potential of blood-based cfDNA tests is immense, several challenges were discussed:
- Detection of Advanced Adenomas: These precancerous lesions may not release sufficient ctDNA for reliable detection, limiting the effectiveness of blood-based tests.
- Tumor Size and Stage: The amount of ctDNA in the bloodstream is generally proportional to tumor size and stage, affecting the sensitivity of these tests.
- Stool-Based Tests: Continue to show superior sensitivity for detecting advanced adenomas compared to blood-based methods.
The Role of Artificial Intelligence
Artificial Intelligence (AI) is making significant inroads into CRC screening, particularly in enhancing the accuracy of colonoscopies. AI-assisted colonoscopy can improve adenoma detection rates by identifying additional neoplastic lesions that might be missed by the human eye.
Future Directions: Fecal MicroRNAs and Multi-Cancer Early Detection
Emerging research into fecal microRNAs presents a promising frontier for CRC detection. These small, non-coding RNA molecules are stable in stool samples and can accurately classify CRC patients from controls. Additionally, Multi-Cancer Early Detection (MCED) tests using cfDNA are being developed to screen for multiple cancer types simultaneously, although their sensitivity for early-stage CRC currently stands at around 53%.
Conclusion
The congress provided valuable insights into the evolving landscape of CRC screening. While traditional methods like colonoscopy and stool-based tests remain highly effective, emerging technologies such as blood-based biomarkers and AI-assisted colonoscopy offer exciting possibilities for the future. As medical professionals, our goal is to leverage these advancements to improve patient outcomes and adherence to screening recommendations. Ultimately, the best screening test is the one that gets done, ensuring that more individuals can benefit from early detection and intervention.
References
- Sinicrope, F. A. (2024). Next Generation Screening for Colorectal Cancer.
- Imperiale TF, et al. NEJM 2024.
- Chung, D et al. NEJM 2024.
- Hassan et al, Gastroenterology 2021.
- Pardini, B et al. Gastroenterology 2022.